usp class vi vs fda
Certificates of Analysis COAs report the test results for a specific batch of materials. Moulded O-rings class 1 less than 10 furnace black These can be produced in all possible dimensions up to diameter 1400 mm internal.

O Rings Fda And Usp Class Vi Darcoid Rubber Company Oakland California
Pharmacopeia a private non-government organization that promotes the public health by establishing state-of-the-art standards to ensure the quality of medicines and other health care technologies.

. USP Class VI approval tests are one of the important standards set by the FDA for products and processes in the food processing industry. Table 1 shows our standard programme FDA compliant com- FDA and USP class VI compliant. FDA food-grade rubber materials typically comply with FDA 21.
Sil 714001 USP class VI Silicone 1 70 Yes transl. Class VI materials which were discussed earlier are tested according to the above protocols. Class VI is the most stringent and requires.
Darcoid and Parker offer a wide range. It can also withstand strict sanitation requirements and its white stain-resistant. It consists of 3 testing requirements.
The United States Pharmacopeia USP is a non-governmental not-for-profit public health organization that is an official public standards-setting authority for all prescription and over-the-counter medicines and other health care products manufactured or sold in the United States. However Class VI also requires subacute toxicity and implantation effects which many ISO 10993 categories do not. Specialty Silicone Products SSP provides complete certifications to demonstrate the quality of its SSP-2390 Series USP Class VI FDA and RoHS compliant silicones.
One standard often overlooked but usually published alongside USP Class VI is FDA 21 CFR 1772600. For plastics they have six different classes based on duration and application. When evaluating a new product many of our customers immediately jump to USP Class VI approval tests.
There may be some confusion between FDA USP Class VI and FDA food grade materials. USP Class VI Chapter 88 relates to in vivo biological reactivity tests its purpose is to determine the biological response impact of elastomeric materials on live animals. USP Class VI testing is conducted by producing an extract of the product with different extraction fluids such as polyethylene glycol and vegetable oil and injecting it in specimen rabbits and mice in vivo alive to observe the biological response to the extract.
Sample of the compound is prepared with specific extraction fluids like vegetable oil and polyethylene glycol. USP stands for US. USP Class VI and FDA White List Silicone and Organic Elastomer Compounds for Healthcare Products.
Sil 714002 USP class VI Silicone 1 70 Yes transl. 27 rows The USP Class VI compounds must be made from ingredients with clear histories of. Consumers implicitly rely upon the standards put into place by governing agencies to protect the publics health and well-being.
Its possible that a USP Class VI material can also. Testing is commonly done as per USP which requires three types of. So does ISO 10993.
USP Class VI demands an intracutaneous irritation test. This post discusses FDA CFR 21 1772600 which is an important aspect of these USP Class VI approval. Specially formulated for long term sealing.
Rulon 641 the only filled PTFE material with USP Class VI approval for direct contact with human tissue and fluids is a powerhouse in the medical and food processing industries. Certificates of Conformance COC attest to a batchs compliance with those requirements. That said the lack of risk assessment in USP Class VI can be a problem.
It offers excellent load and wear resistance plus good chemical resistance. Both ISO 10993 and USP Class VI define testing requirements for biocompatibility the ability of a material to perform a desired function without causing adverse effects on the human body. These standards define the purity and reliability of a product or process and prioritize public wellbeing.
USP Class VI vs. USP Class VI materials EPDM Silicone Fluorocarbon and Perfluoroelastomer 24 materials which are compliant to FDA 21 CF R1772600. While it is possible a USP Class VI material could also be ISO 10993 compliant its not a given and USP Class VI alone is not sufficient for adherence to ISO 10993.
That being said if you cant get an ISO 10993 compliant material often because the material simply hasnt been tested using a USP Class VI material is a less risky option. The USP also establishes standards for food ingredients.

Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa

0 50 1 2 Id Fda Usp Class Vi Platinum Silicone W Polyester Braid Food And Pharma Grade Flex Technologies Incorporated

Usp Class Vi Foster Corporation
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Understanding Food Grade Vs Biocompatibility For Medical Device Materials Medical Product Outsourcing

Fda And Usp Class Vi O Rings Guide 2020 Nes

Rulon 641 Usp Approved And Fda Compliant Tristar Plastics
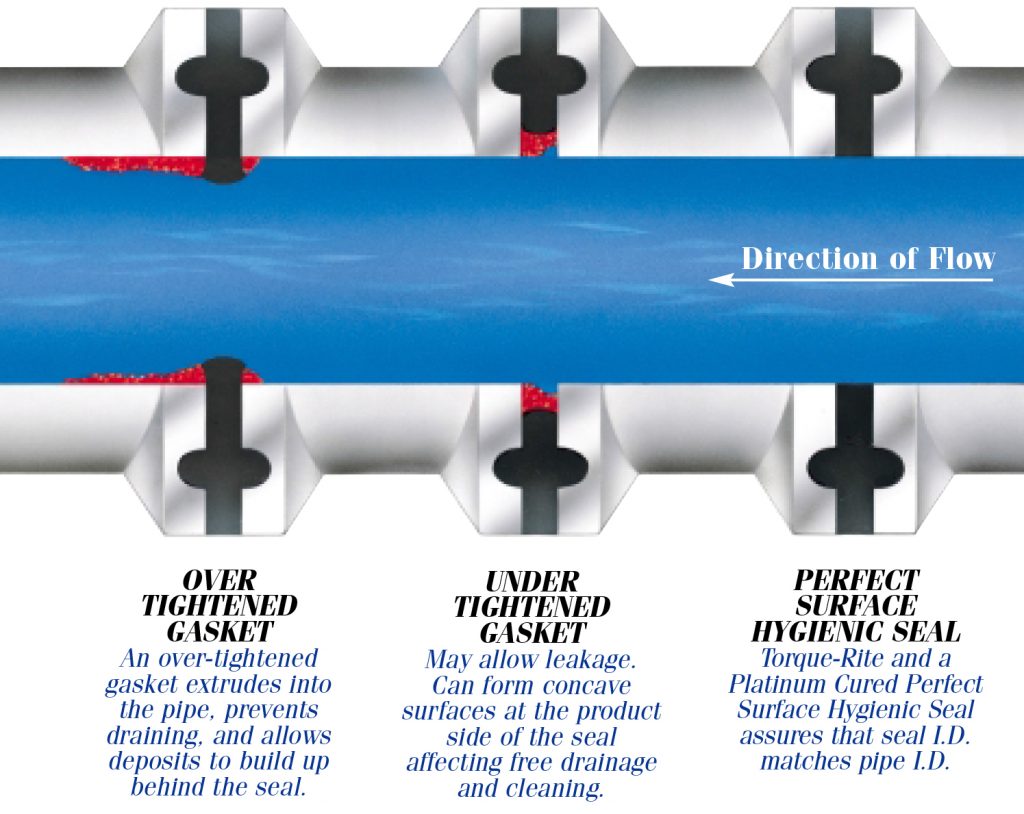
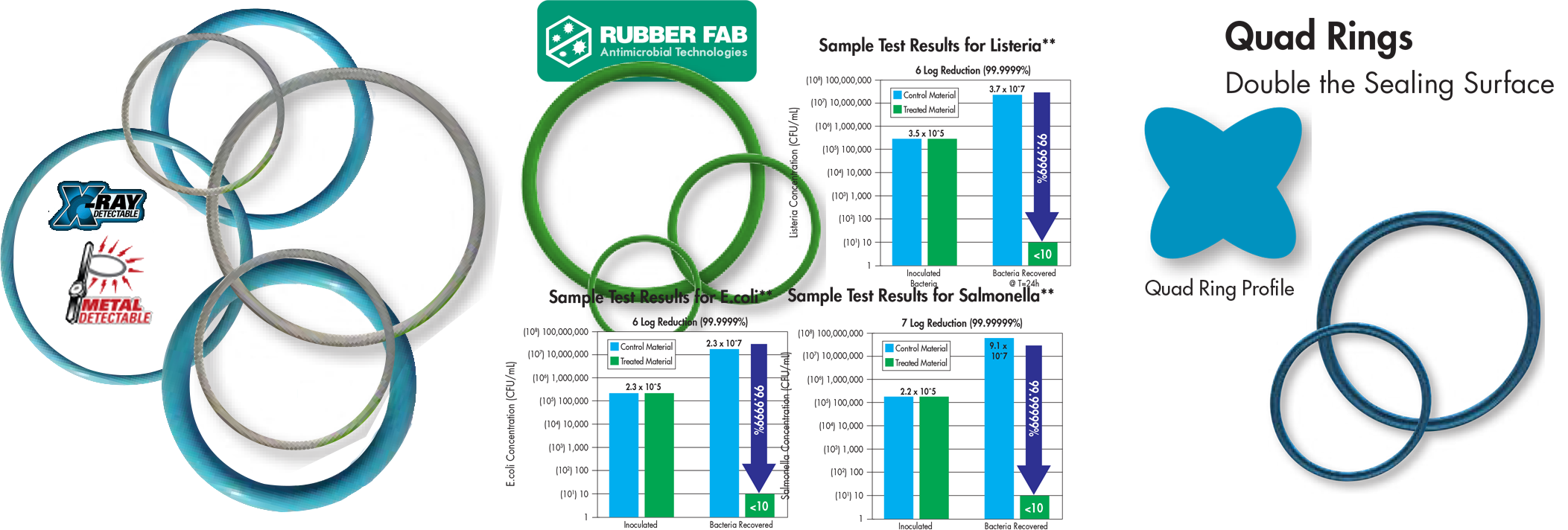
Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa

Duraform Pa Certification Usp Class Vi Iso 10993 And Food Contact
![]()
Usp Class Vi Silicone Is Independently Certified For Biocompatibility Specialty Silicone Products Inc
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

Material Selection Medical Injection Molding Xcentric Mold

Fda Usda Nsf51 Usp Class Vi Compliant Seals Products

Parker V1274 75 Usp Class Vi Biocompatibility O Ring United Seal